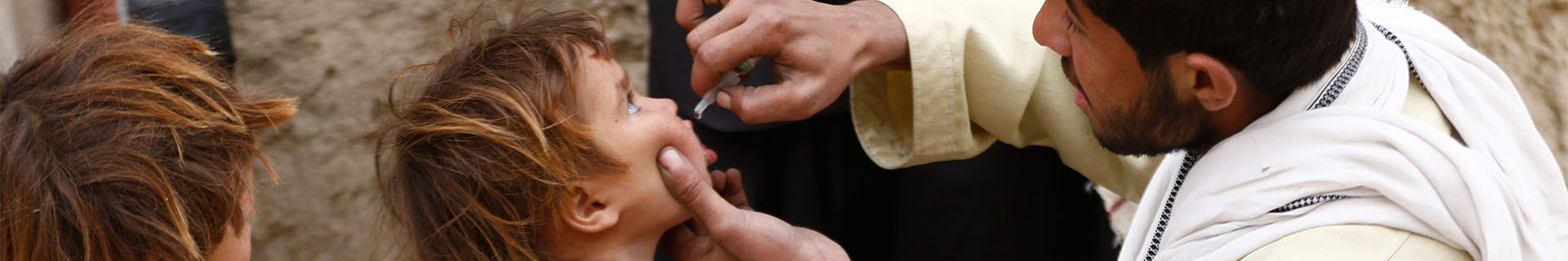
nOPV2
To better address the evolving risk of type 2 circulating vaccine-derived poliovirus (cVDPV2), GPEI partners together with countries are deploying an innovative tool – novel oral polio vaccine type 2 (nOPV2). The vaccine is a modified version of the type 2 monovalent OPV (mOPV2), which clinical trials have shown provides comparable protection against poliovirus while being more genetically stable and less likely to be associated with the emergence of cVDPV2 in low immunity settings. This means that nOPV2 has the potential to be a significant tool to help stop outbreaks more sustainably.
nOPV2 is being deployed under WHO’s Emergency Use Listing procedure (EUL) to enable its rapid field availability. Even after meeting rigorous EUL criteria for safety and immunogenicity, nOPV2’s performance in the field is being closely monitored in line with EUL standards and data collection continues with the ultimate goal of WHO prequalification and full licensure.
Key Resources
GPEI nOPV2-cVDPV2 fact sheet | English | French |
PATH fact sheet: Advancing novel polio vaccines | English |
nOPV2 Frequently Asked Questions (FAQs) | English |
nOPV2 technical brief | English |
Novel oral polio vaccine type 2 (nOPV2) animation video | English | French | Portuguese | Spanish |
WHO Emergency Use Listing procedure infographic | English | French |
Clinical summary for novel oral polio vaccine type 2 (nOPV2) |English | French | Arabic | Portuguese |
Scientific publications on nOPV2:
-
- Assessment of genetic changes and neurovirulence of shed Sabin and novel type 2 oral polio vaccine viruses (2021 npj Vaccines publication)
- Engineering the Live-Attenuated Polio Vaccine to Prevent Reversion to Virulence(2020 Cell Host & Microbe publication)
- Development of a new oral poliovirus vaccine for the eradication end game using codon deoptimization(2020 npj Vaccines publication)
- Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in healthy adults: two clinical trials (2020 Lancet publication)
- Safety and immunogenicity of two novel type 2 oral poliovirus vaccine candidates compared with a monovalent type 2 oral poliovirus vaccine in children and infants: two clinical trials (2020 Lancet publication)
- The safety and immunogenicity of two novel live attenuated monovalent (serotype 2) oral poliovirus vaccines in healthy adults: a double-blind, single-centre phase 1 study(2019 Lancet publication)
- Polio endgame options: will we have the vaccines needed? (2019 Lancet commentary)
- Poliopolis(2019 correspondence in The Lancet)
- Poliopolis: pushing boundaries of scientific innovations for disease eradication(2019 Future Microbiology publication)
- Polio vaccination: past, present, and future(2015 Future Microbiology publication)
- Intestinal antibody responses to two novel live attenuated type 2 oral poliovirus vaccines in healthy adults in Belgium (2021 Journal of Infectious Diseases publication)
nOPV2 Vaccine Management Guidance | English |
nOPV2 Vaccine Handling | English |
nOPV2 Surveillance Guidance | English |
nOPV2 Safety Guidance | English |
nOPV2 AESI guidance | English |
GPEI polio outbreak response training | English |
Introduction of nOPV2 for Polio Outbreak Response: Supervisory manual | English | French |
GPEI position statement: use of the novel oral polio vaccine type 2 in light of shelf-life of 12 months or less | English |
Interim Guidance on the use of Novel Oral Polio Vaccine Type 2 (nOPV2) for the response to Type 2 Circulating Vaccine-Derived Poliovirus (cVDPV2) during the Initial Use Period (Addendum to the Standard Operating Procedures for Response to a Poliovirus Event or Outbreak, version 3. 1) | English | French | Portuguese | Russian |
Novel oral polio vaccine type 2 (nOPV2) Vaccine-Related Event (VRE) Response Plan | English | French | Arabic | Portuguese |
VRE development guidance note for countries | English | French | Portuguese |
UNICEF Strategic C4D Communications Guidance for Outbreak Response including nOPV2 | English |
Programme Advocacy package for cVDPV Outbreak Response and nOPV2 Introduction | English |
Vaccine Misinformation Management Field Guide | English |
Highlights from the Meeting of the Strategic Advisory Group of Experts (SAGE) on Immunization 4-7 October 2021 |English |
Framework for the Initial Use of nOPV2, endorsed by the SAGE | English | French |
WHO Executive Board decision urging Member States to expedite processes for authorizing the importation and use of nOPV2 on the basis of its emergency use listing – February 2020 | English| French|
SAGE Recommendations – June 2021 Meeting (Weekly Epidemiological Record) – English and French
SAGE Recommendations – October 2020 Meeting (Weekly Epidemiological Record) – English and French
SAGE Recommendations April 2020 Meeting (Weekly epidemiological record) – English and French
SAGE Recommendations, October 2019 Meeting (Weekly epidemiological record) – English and French
Media center
- Independent experts advise move to next use phase for novel oral polio vaccine type 2
- Countries gear up to kick all forms of polio out of Africa, once and for all
- Recommendations for reporting on Poliovirus outbreaks | English | French |
- Novel Oral Polio Vaccine type 2 (nOPV2) granted interim Emergency Use Listing recommendation
- Recommandation provisoire d’autorisation d’utilisation d’urgence pour un nouveau vaccine
- Special edition of Polio News – May 2020
Additional Resources and Updates
- Strategy for the Response to Type 2 Circulating Vaccine-Derived Poliovirus 2020–2021 |English |
- nOPV2 Working Group:
- GACVS sub-committee on novel OPV2 safety